US drug agency revokes emergency use status of Trump-touted coronavirus medicine
US FDA said it no longer believed based on new evidence that hydroxychloroquine and chloroquine may be effective in treating coronavirus
June 15, 2020
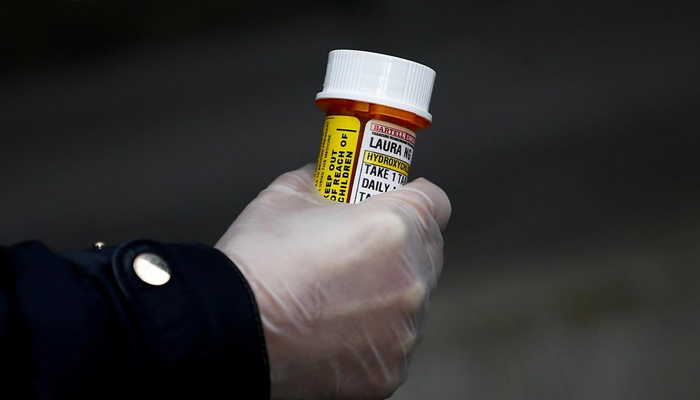
WASHINGTON: The top US drug regulatory body on Monday revoked its emergency use authorisation for hydroxychloroquine to treat coronavirus, a drug that US President Donald Trump touted would stave off the infection.
Based on new evidence, the US Food and Drug Administration (FDA) said it was no longer reasonable to believe that oral formulations of hydroxychloroquine and the related drug chloroquine may be effective in treating the illness caused by the novel coronavirus.
The move comes after several studies of the decades-old malaria drug suggested it was not effective, including a widely anticipated trial earlier this month that showed it failed to prevent infection in people who had been exposed to the virus.
The drug’s anti-inflammatory and antiviral properties suggested it might help treat COVID-19, and the FDA authorised its emergency use in March at the height of a pandemic for which there were no approved treatments.
While it did appear to neutralise the virus in laboratory experiments, hydroxychloroquine, which is also used to treat lupus and rheumatoid arthritis, has failed to prove its worth in human COVID-19 trials, thus far.
In March, Trump said hydroxychloroquine used in combination with the antibiotic azithromycin had “a real chance to be one of the biggest game changers in the history of medicine,” with little evidence to back up that claim.
He later said he took the drugs preventively after two people who worked at the White House were diagnosed with COVID-19, and he urged others to try it.
Doctors in recent weeks had already pulled back on the use of hydroxychloroquine as a COVID-19 treatment, after several studies suggested it is not effective and may pose heart risks for certain patients.
Current US government treatment guidelines do not recommend use of the malaria drugs for COVID-19 patients outside of a clinical trial.
France, Italy and Belgium late last month moved to halt the use of hydroxychloroquine to treat COVID-19 patients. But the United States last month supplied Brazil with 2 million doses for use against the coronavirus, as the South American country has emerged as the pandemic’s latest epicenter.
Meanwhile, some 400 trials are listed as using hydroxychloroquine or chloroquine as interventions for COVID-19, more than half of them still ongoing, according to a recent analysis from research firm GlobalData.
In the US, the National Institute of Allergy and Infectious Diseases last month launched a trial designed to show whether hydroxychloroquine in combination with azithromycin can prevent hospitalisation and death from COVID-19.