Safety lapses, weak oversight: How children die from Indian cough syrup
Tamil Nadu’s health officials believe solvent, tainted with toxic chemical, used in cough syrup
November 21, 2025
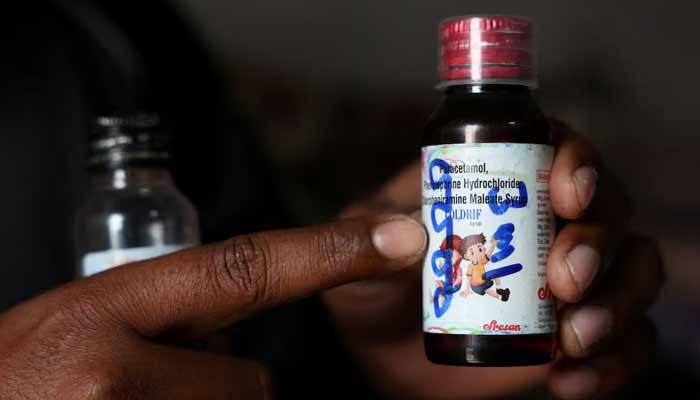
Indian officials are investigating whether safety lapses in the supply of a pharmaceutical ingredient were responsible for contaminating cough syrup that has killed at least 24 children in recent months, according to three people familiar with the matter.
The three health and drug safety officials from Tamil Nadu state told Reuters they believe the solvent used to make a batch of Coldrif cough syrup could have been contaminated with a toxic chemical around the time it was supplied to the drugmaker, Sresan Pharmaceutical Manufacturer.
Sresan acquired 50 kg of the propylene glycol (PG) solvent from local chemicals distributor Sunrise Biotech on March 25, which had purchased it the same day from Jinkushal Aroma, a small company that makes fragrance blends for liquid detergents and other chemicals, according to interviews with the suppliers and an October 3 investigation report by the Tamil Nadu pharmaceutical regulator, exclusively seen by Reuters.
The Tamil Nadu Drugs Control Department didn't respond to repeated requests for comment about its investigation.
Authorities have said the Coldrif syrup was heavily contaminated with a known industrial toxin, diethylene glycol (DEG). They are investigating how the chemical was added to the solvent, which is used in cough syrup as a base for dissolving its active ingredients.
The fatalities, which began in September, have revived concerns about safety standards in India's $50 billion pharmaceutical sector, which was tarnished by the deaths of more than 140 children in Africa and Central Asia in 2022 and 2023 from Indian-made cough syrups made with contaminated solvents.
In the wake of those deaths, New Delhi had pledged to improve quality controls.
Indian health officials say DEG is sometimes fraudulently or unintentionally used in medicines in place of pricier PG. Ingesting high levels of it has been linked to acute kidney damage and death in children.
Reuters is reporting details for the first time about the focus of the Indian investigation, as well as breaches in global pharmaceutical safety practices in the delivery of the chemicals to Sresan.
Sresan's manufacturing licence has been revoked and its founder, G. Ranganathan is in custody. Efforts to contact representatives at Sresan's corporate office and Ranganathan's home were unsuccessful. Reuters was unable to identify a legal representative for Ranganathan.
The Central Drugs Standard Control Organisation, which oversees pharmaceuticals federally, directed questions to India's health ministry, which in turn referred Reuters to a government statement saying it was conducting more inspections of drug facilities and reviewing pediatric use of cough syrups.
Chemical makers typically deliver PG solvents to clients in sealed containers to avoid contamination, but Sunrise confirmed to Reuters that it had repackaged the solvent without a seal before delivering it.
India's Drugs and Cosmetics Act prohibits the sale and handling of pharmaceutical-grade ingredients like medicinal PG by entities that do not have drug licences.
Neither Jinkushal nor Sunrise have licences for handling pharmaceutical-grade ingredients, the two wholesale distributors confirmed to Reuters. Their owners said they weren’t aware the PG they had sold would be used to make medication.