BF Biosciences in talks to manufacture COVID-19 drug in Pakistan
Gilead Sciences had earlier said it was negotiating long-term voluntary licenses with several generic drugmakers in Pakistan to produce remdesivir for developing countries
May 12, 2020
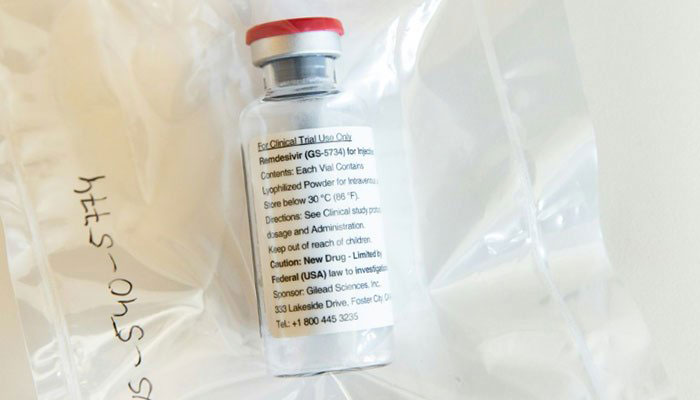
KARACHI: Ferozsons Laboratories have announced that its listed subsidiary BF Biosciences Limited (BFBL) has been negotiating for a non-exclusive license agreement with Gilead Sciences to manufacture and sell remdesivir to supply in Pakistan and 126 other countries, reported The News.
A statement issued by Ferozsons Laboratories said BFBL had been negotiating but there has been no obligation at this time for any part to execute any transactions.
Earlier this month, the California-based drug manufacturer had said it was negotiating long-term voluntary licenses with several generic drugmakers in Pakistan and India to produce experimental COVID-19 drug remdesivir for developing countries.
The drugmaker also said it was in discussions with chemical and drug manufacturers to produce its antiviral drug remdesivir for Europe, Asia and the developing world through at least 2022.
In April, Gilead had received emergency authorisation from the US Food and Drug Administration for using remdesivir as a treatment against COVID-19.
The approval was the latest step in a global push to find viable treatments and a vaccine for the coronavirus.
The company has previously announced it was donating some 1.5 million doses for free. This amounts to about 140,000 treatment courses based on 10-day treatment duration.
Read also: Remdesivir maker in talks with Pakistani, Indian firms to produce COVID-19 drug
Remdesivir, which is administered through an injection, was already available to some patients who enrolled in clinical trials, or who sought it out on a "compassionate use" basis.
The Food and Drug Administration, which authorised the approval, defines severe as having low blood oxygen levels, requiring oxygen therapy, or being on a ventilator.
In a trial involving more than 1,000 people, the US National Institute of Allergy and Infectious Diseases (NIAID) found that hospitalised COVID-19 patients with respiratory distress got better quicker than those on a placebo.
Specifically, patients on the drug had a 31% faster time to recovery.
"Although the results were clearly positive from a statistically significant standpoint, they were modest," Anthony Fauci, the scientist who leads the NIAID had told NBC News.
While not considered a miracle cure, remdesivir's trial achieved a "proof of concept," according to Fauci that could pave the way for better treatments.
Remdesivir incorporates itself into the virus's genome, short-circuiting its replication process.
It was first developed to treat Ebola, a viral hemorrhagic fever, but did not boost survival rates as other medicines.