WHO sets dates for reviews of Sinopharm and Sinovac COVID-19 vaccines
So far, COVID-19 vaccines made by Pfizer AstraZeneca and Johnson & Johnson have received a WHO listing
April 23, 2021
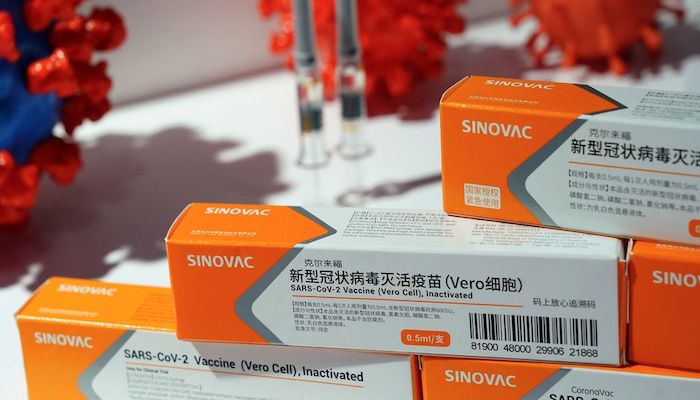
- WHO to review Chinese coronavirus vaccines Sinopharm and Sinovac for emergency use listing.
- A WHO listing is an endorsement of a vaccine's safety and efficacy that helps to guide countries’ regulatory agencies.
- The two China-made vaccines have seen strong demand from many developing countries.
Technical experts at the World Health Organization (WHO) will review on April 26 Chinese drugmaker Sinopharm’s COVID-19 vaccine for possible emergency use listing, to be followed by the Sinovac jab on May 3, the agency said on Thursday.
“We would expect a decision a couple of days later,” the WHO said in response to a Reuters query.
So far, COVID-19 vaccines made by Pfizer AstraZeneca and Johnson & Johnson have received a WHO listing — an endorsement of their safety and efficacy that helps to guide countries’ regulatory agencies.
Read more: Johnson & Johnson to resume rollout of coronavirus vaccine in Europe with safety warning
No detailed efficacy data of Sinopharm’s COVID-19 vaccine has been publicly released but its developer, Beijing Biological Products Institute, a unit of Sinopharm subsidiary China National Biotec Group (CNBG), said the vaccine was 79.34% effective in preventing people from developing the disease based on interim data.
It has been approved in several countries including China, Pakistan and the UAE.
Sinovac’s vaccine showed varied efficacy readings of between 50.65% and 83.5% based on trials from Brazil, Turkey and Indonesia.
Read more: 500,000 SinoVac coronavirus vaccine doses arrive in Pakistan
The two China-made vaccines have seen strong demand from many developing countries which have limited access to shots made by rival Western drugmakers.