India orders 300 million COVID-19 shots before approval
The government will buy 300 million doses from local firm Biological-E and has put down an advance of $205.6 million, says the health ministry
June 03, 2021
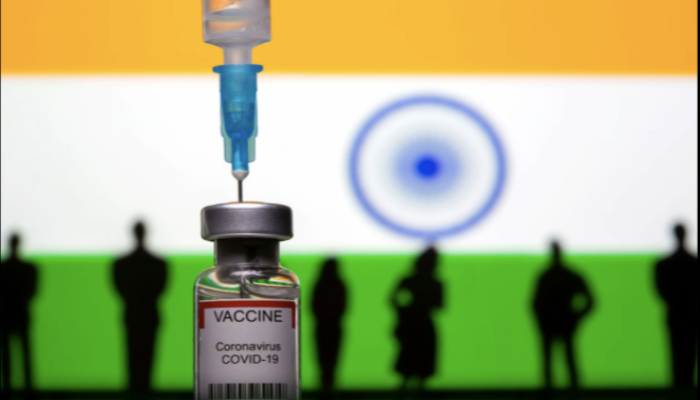
- India's government signed its first purchase order for unapproved COVID-19 vaccines on Thursday.
- After the first wave of coronavirus, the focus has shifted to urgently inoculating India's vast adult population to curb infections later this year.
- The government will buy 300 million doses from local firm Biological-E and has put down an advance of $205.6 million, says the health ministry.
NEW DELHI: A day after the Indian government drew flak from the top judiciary about a bungled vaccine rollout that has left millions of people vulnerable, India's government signed its first purchase order for unapproved COVID-19 vaccines on Thursday.
After a devastating second wave of infections that killed tens of thousands in April-May, the focus has shifted to urgently inoculating India's vast adult population to curb infections later this year.
The government will buy 300 million doses from local firm Biological-E and has put down an advance of $205.6 million, the health ministry said, even though the vaccine is still undergoing phase-3 clinical trials before approvals can be given.
"The arrangement with M/s Biological-E is part of the wider endeavour of Government of India to encourage indigenous vaccine manufacturers by providing them support in Research & Development (R&D) and also financial support," the ministry said in a statement.
India has been inoculating its people with the AstraZeneca vaccine produced locally at the Serum Institute of India (SII), Covaxin made by local firm Bharat Biotech and has begun rolling out Russia's Sputnik V.
But supplies are running tight after the government opened vaccinations to all adults last month. Some vaccination centres have had to close down, prompting criticism from the Supreme Court about a lack of proper planning.
Read more: India records over 134,000 new coronavirus cases over past 24 hours
While the federal government gave free vaccines to the elderly and frontline workers, it left it to state governments and private hospitals to administer doses to people in the 18 to 45 age group at a pre-determined price.
"The policy of the central government for conducting free vaccination themselves for groups under the first 2 phases, and replacing it with paid vaccination...is, prima facie, arbitrary and irrational," the Supreme Court said.
Younger people were just as vulnerable, as the second wave of infections had shown, the court said.
It asked the government to review its vaccination policy, produce a roadmap, and said the court could not be a silent spectator when the constitutional rights of the citizens were at risk.
So far, about 4.7% of the country's 950 million adult population have been given both doses.
The government said this week supplies are improving and it could have as many as 10 million doses each day in July and August, up from just under three million now.
The Serum Institute of India has sought regulatory approval to make Russia's Sputnik V COVID-19 vaccine, a senior government official said, on top of the AstraZeneca and Novavax Inc shots it is already producing.
The federal drugs regulator did not immediately respond to a Reuter's e-mail seeking comment.
India announced on Thursday 134,154 new COVID-19 infections over the past 24 hours, still high but down more than 65% from a peak of 414,188 reported on May 7.
The officially recorded caseload since the start of the pandemic now stands at 28.4 million.