Pakistan likely to receive Moderna COVID-19 vaccine in coming months: health ministry officials
So far, Pakistan has received 100,600 doses of Pfizer-BioNTech’s mRNA vaccine through COVAX
June 25, 2021
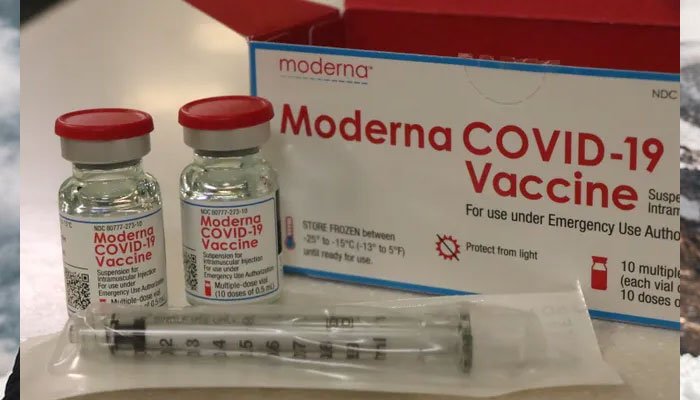
- Talks underway to get Moderna's mRNA-1273 vaccine through COVAX, say officials from Pakistan's health ministry.
- But officials say no confirmation so far on when and how many doses of Moderna vaccine would be provided to Pakistan.
- Drug Regulatory Authority of Pakistan has yet to grant 'emergency use' authorisation to Moderna coronavirus vaccine.
KARACHI: Pakistan is likely to receive an "unspecified number" of Moderna’s mRNA-1273 coronavirus vaccine doses in the coming months, say officials from the country's health ministry
Talks are ongoing with COVAX to acquire the Moderna vaccine, officials in the Ministry of National Health Services Regulations and Coordination told The News.
But the officials said there has so far been no confirmation on when and how many doses of the double-dose vaccine would be provided to Pakistan.
Getting a coronavirus vaccine in Pakistan: What you should know
So far, Pakistan has received 100,600 doses of Pfizer-BioNTech’s mRNA vaccine through COVAX for inoculation of immunosuppressant people.
The federal government and Pfizer-BioNTech have also reached an agreement for the supply of 13 million more doses of the mRNA vaccine by 2021 end.
If Moderna’s vaccine is made available through COVAX, it would be a valuable addition to the existing Chinese and European vaccines, officials said.
A large number of doses, however, are not expected to be supplied by COVAX, the officials clarified, adding that the Moderna vaccine supply has no co-relation with Prime Minister Imran Khan's recent conversation over the phone with Bill Gates.
Read more: Pakistan revises guidelines for two-dose Chinese vaccines
The Drug Regulatory Authority of Pakistan (DRAP) has yet to grant 'emergency use' authorisation to the Moderna coronavirus vaccine.
DRAP officials said they have conveyed the legal requirements for import of vaccines.
“If a vaccine is allowed for emergency use by the US Food and Drug Administration and European Medicine Agency, there is no need for emergency use authorisation from DRAP,” a DRAP official said.
He said, however, that if Moderna or any of its agents want to get the vaccine registered in Pakistan, they can apply for the emergency use authorization with DRAP like Chinese, US and other European vaccine manufacturers have done.
Pakistan has so far granted emergency use authorization to six different vaccines, including China’s Cansino, Sinopharm, Sinovac, Russian Sputnik V, European AstraZeneca and US Pfizer vaccines.
Read more: 1.55mn doses of Sinovac vaccine arrive in Pakistan